Limescale doesn’t just form on taps and shower heads. Limescale can form on any surface where water is heated, including the internal surfaces of pipework and boilers.
Find out how Combimate can prevent the build-up of limescale and save you money on heating and costly repair bills.
Your home is vulnerable to the damaging effects of limescale formation in a variety of places:


Limescale is the most visible symptom of hard water. It is comprised of calcium and magnesium, two minerals that form a residue when hard water is heated and evaporates.
This residue forms as a white chalky scum, commonly found around taps, the bath, basins and shower cubicles. Limescale is an unwanted substance and is difficult to remove.
Inside a kettle is the most obvious place to spot limescale – the process of ‘descaling’ is common practice to restore efficiency and remove unwanted scale build-up.
However, limescale also builds-up in places you can’t see. Left unchecked this scale will steadily reduce a home’s heating efficiency by clogging up pipes and boiler heat exchangers. This leads to decreased water pressure, flow rates and an increased energy demand; in turn leading to higher energy bills and maintenance costs.
For more information on how to reduce energy bills by preventing scale formation, please see the article in our User Guides section.
Approximately 60% of the UK lies within a hard water area.
British Water, the trade association for the UK water industry, has calculated that hard water, used by an average family of four, accumulates 70kg of limescale a year.
In hard water areas, limescale can build-up on the waterside of the boiler’s heat exchanger. This creates an insulating layer, inhibiting heat transfer to the water. Limescale builds in hard water conditions at a rate of about 1mm a year.
British Water calculate that every 1.6mm or 1/16” of scale in a heating system causes a 12% loss in heating efficiency.
The Carbon Trust, which advises on energy efficiency, report similar findings: “A 1mm layer of limescale will cause a 7% increase in energy input to the boiler to meet the same heat demand.”
Energy wastage is only part of the equation. Scaling can cause premature failure of boiler heat exchangers – this part then needs to be replaced and often whole boilers are written-off and new units required.
The carbon footprint of both actions is considerable: the procurement of raw materials, processing the components and the assembly and transportation of the replacement units across the globe.
Domestic energy consumption – The Government estimates that 80% of the average home’s total annual energy consumption goes on heating (space and water combined).
This equates to approximately 15,600 kWh at a cost of approximately £1,950. Much of this involves domestic boilers heating water to use directly and to fill central heating systems.

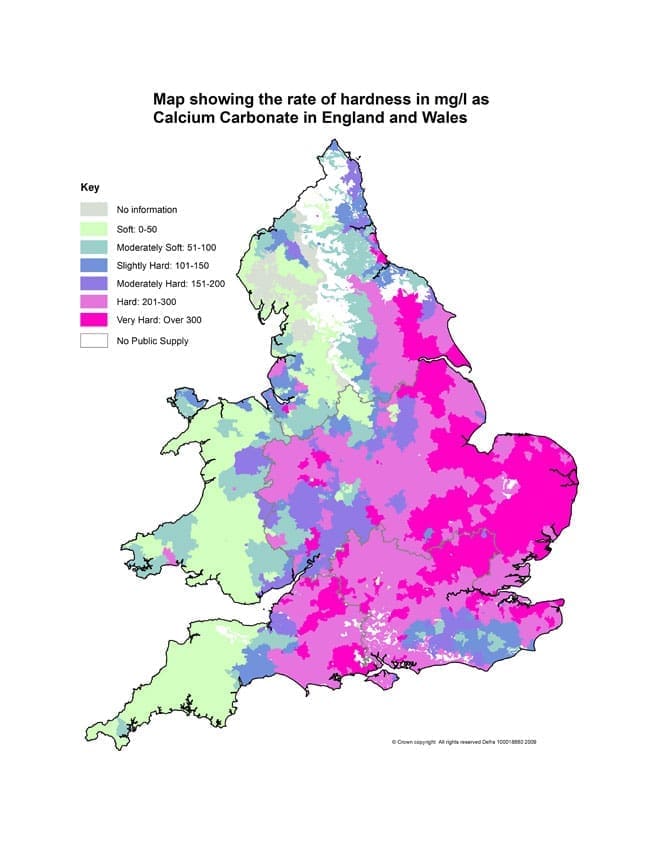
Water hardness levels vary across the UK but the majority of the country (approximately 60%) lies within a hard water area.
In England & Wales water hardness varies by region. In particular, parts of eastern England have an extremely hard water supply. Very hard water is considered to be over 300ppm (parts per million).
In Scotland most water supplies are classified as soft. Chalk or limestone rocks (containing the greatest amount of calcium and magnesium minerals) are not common in Scotland, which benefits from naturally sourced soft water from river, highland areas and reservoirs.
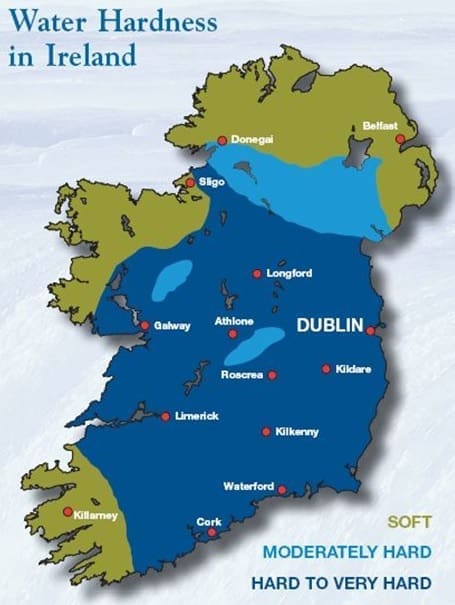
In Northern Ireland and the Republic of Ireland water hardness varies markedly by region. The majority of Northern Ireland has soft water and a significant proportion of the Republic of Ireland has a hard water supply.
Your household water supply is drawn from the regional environment. After rainwater has fallen on the ground it collects hard minerals as it percolates down through rocks such as chalk and limestone. These minerals make the water hard.
The amount of mineral deposits in your water (and therefore the hardness of the water) will depend on which area of the country you live in.
In the home the results of hard water are the limescale deposits that it leaves behind.
It can be a surprise to learn that naturally occurring soft water can be harmful to your water system.
Natural soft water is water that contains very little natural minerals and this ’pure’ water is actually aggressive to metals and causes corrosion. The symptoms of such corrosion can be when the residue (caused by oxidisation of the metal pipes) comes out of your taps, turning tap water brown. Left unchecked, such continual corrosion will cause pinholes in the pipe and leaks may be the result.
Natural soft water (sources from lakes, rivers etc.) is often low in pH (a term used to describe a scale of acid/alkalinity) making it slightly acidic. This acidity makes the water corrosive to metals.
Hard water can still be acidic, but certain minerals in the water have properties that prevent the chemical reaction of corrosion taking place – thus providing a level of protection for the metal. The drawback is that such minerals can also build-up as limescale.


These devices have been widely accepted as a solution to combat the effects of limescale by removing minerals in the hard water supply.
Water softeners employ differing methods of operation (depending on model), but there is a long-running debate as to whether hard water that has been artificially softened is corrosive to heating systems or not.
Domestic water softeners also come with their own pros and cons. For more information on water softeners, please consult the article in our User Guides section.
Combimate beats both limescale and soft water corrosion.
Combimate is not a water softener but a phosphate dosing device. Combimate does not alter the hardness (or softness) of the home’s water supply but, through the process of adding phosphate, prevents limescale build-up and provides soft water corrosion protection.
For product support or installation advice, contact our dedicated support staff today.
